Selective synthesis of pyrrolidin-2-ones and 3-iodopyrroles via the ring contraction and deformylative functionalization of piperidine derivatives†
Abstract
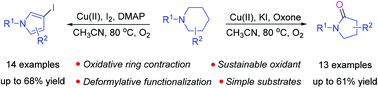
In this paper, a selective synthesis of pyrrolidin-2-ones and 3-iodopyrroles via the cascade reactions of N-substituted piperidines is presented. Mechanistically, the formation of pyrrolidin-2-ones involves a domino process including the in situ formation of pyrrolidine-2-carbaldehyde followed by carboxylic acid formation, decarboxylation and ipso-oxidation. On the other hand, 3-iodopyrroles are believed to be formed via the initial generation of pyrrolidine-2-carbaldehyde followed by carboxylic acid formation, decarboxylation, dehydrogenation, iodination and aromatization. Interestingly, either pyrrolidin-2-ones or 3-iodopyrroles could be obtained selectively from the same substrates, and the selectivity was easily tuned by using a specific oxidant and additive.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...