Cyclopropanation of alkenes with metallocarbenes generated from monocarbonyl iodonium ylides†
Abstract
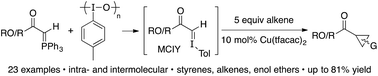
Reacting Wittig reagents and the hypervalent iodine reagent iodosotoluene, in the presence of 10 mol% Cu(tfacac)2 and 5 equiv. of alkene, results in a novel cyclopropanation reaction. The reagent combination is believed to generate a transient monocarbonyl iodonium ylide (MCIY) in situ, which can be intercepted by the copper catalyst to give a metallocarbene. Both ester and ketone derived phosphoranes can be used, as can styrenyl and non-styrenyl alkenes, which provides cyclopropanes in yields up to 81%.
- This article is part of the themed collections: Synthetic methodology in OBC and Carbenes in Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...