Synthesis of quinazoline-3-oxides via a Pd(ii) catalyzed azide–isocyanide coupling/cyclocondensation reaction†
Abstract
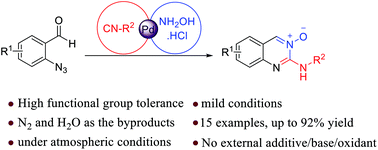
A novel and efficient protocol concerning palladium catalyzing the three-component reaction of 2-azidobenzaldehyde, isocyanide, and hydroxylamine hydrochloride is developed. This method allows the rapid elaboration of quinazoline 3-oxides in a one-pot fashion. The 3-CR mainly involves concatenation of azide–isocyanide denitrogenative coupling, condensation with hydroxylamine and 6-exo-dig cyclization. The salient features of the methodology are operational simplicity, use of milder reaction conditions, being devoid of any additives such as oxidants (redox neutral) or base, and releasing N2 and H2O as the byproducts.
- This article is part of the themed collections: Catalysis & biocatalysis in OBC and Celebrating the RAOBC symposium


 Please wait while we load your content...
Please wait while we load your content...