A unified synthesis of topologically diverse Aspidosperma alkaloids through divergent iminium-trapping†
Abstract
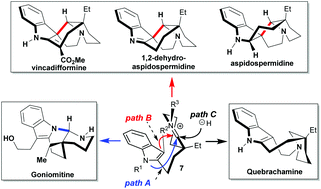
Aspidospermidine, vincadifformine, 1,2-dehydroaspidospermidine, goniomitine, and quebrachamine, five Aspidosperma alkaloids distributed within three structurally diverse topologies, were synthesized from a single molecular scaffold, namely indole–valerolactam 6. This common intermediate can be divergently manipulated, through the incorporation of conformational and electronic constraints that influence the chemo-selectivity of the iminium ion derived therefrom, between three different reaction paths: N(1) vs. C(3) cyclization (indole numbering) vs. over-reduction. Moreover, a catalytic carbene insertion for direct C(3)–H indole functionalization is reported for the first time in an approach to goniomitine (4), and a following tandem ester reduction/iminium generation/cyclization secured its tetracyclic system. The development of a highly diastereoselective one-pot hemi-reduction/cyclization/deprotection process to obtain a cis-pyridocarbazole directly allowed the synthesis of pentacyclic Aspidosperma alkaloids 1, 2, and 3.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...