Synthetic manipulations of polyunsaturated fatty acids as a convenient strategy for the synthesis of bioactive compounds
Abstract
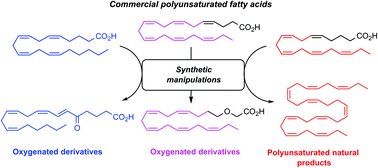
Stereoselective synthesis of Z-configured double bonds is central in organic synthesis due to the presence of such motifs in polyunsaturated fatty acids and many natural products. Traditionally, reductions of internal alkynes or Wittig, Ando or Still–Gennari reactions, are often used for preparing such compounds. The substrate scope is limited for both the Ando and the Still–Gennari reactions, while the Wittig reaction often gives low Z-selectivity for the synthesis of polyunsaturated Z-configured methylene interrupted (skipped) double bonds. Reductions of internal alkynes are challenging due to diminished Z-selectivity, poor catalyst reproducibility and over-reductions. An alternative and highly attractive approach is to employ naturally occurring and commercially available polyunsaturated fatty acids as starting materials. The main advantage of this strategy is the conservation of the multiple Z-configured double bonds present in the starting material, allowing a precise incorporation of the desired double bonds into the final product. In particular, arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid have been used for the stereoselective synthesis of polyunsaturated fatty acids, their derivatives and other polyunsaturated natural products. Herein, such efforts are reviewed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...