A rapid construction of the ABC tricyclic skeleton of malabanone A†
Abstract
The construction of the ABC tricyclic skeleton of malabanone A with the required 4 stereocenters was accomplished in a concise route starting from R-carvone. The synthesis featured an intramolecular [3 + 2] cycloaddition reaction to assemble its A ring and an intramolecular Diels–Alder reaction to construct its C ring.
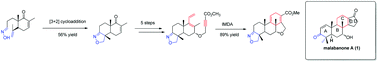


 Please wait while we load your content...
Please wait while we load your content...