Asymmetric synthesis of β-amino ketones by using cinchona alkaloid-based chiral phase transfer catalysts†
Abstract
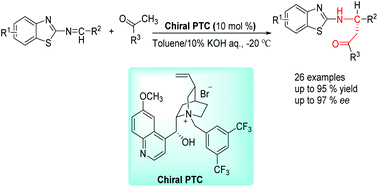
A highly enantioselective nucleophilic addition of ketones to imines catalyzed by chiral phase-transfer catalysts (N-quaternised cinchona alkaloid ammonium salts) has been developed, and the process affords the Mannich reaction products with tertiary stereocenters in good to high yields (up to 95%) with excellent enantioselectivities (up to 97% ee).
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...