Multicomponent dipolar cycloadditions: efficient synthesis of polycyclic fused pyrrolizidines via azomethine ylides†
Abstract
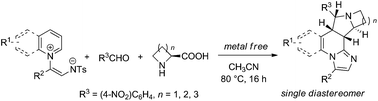
An efficient multicomponent dipolar cycloaddition for the synthesis of polycyclic fused pyrrolizidines was developed using N-aromatic zwitterions, aldehydes, and amino acids. The developed reactions proceed through azomethine ylides generated in situ from the decarboxylated reactions of aldehydes and amino acids followed by the [3 + 2] cycloaddition of N-aromatic zwitterions under mild reaction conditions.
- This article is part of the themed collections: Synthetic methodology in OBC and New Talent


 Please wait while we load your content...
Please wait while we load your content...