Covalent capture of OGT's active site using engineered human-E. coli chimera and intrastrand DNA cross-links†
Abstract
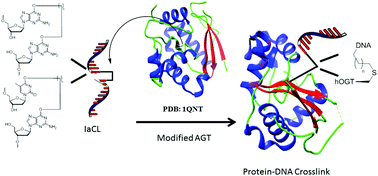
O 6-Alkylguanine DNA alkyltransferases (AGTs) are proteins found in most organisms whose role is to remove alkylation damage from the O6- and O4-positions of 2′-deoxyguanosine (dG) and thymidine (dT), respectively. Variations in active site residues between AGTs from different organisms leads to differences in repair proficiency: The human variant (hAGT) has a proclivity for removal of alkyl groups at the O6-position of guanine and the E. coli OGT protein has activity towards the O4-position of thymine. A chimeric protein (hOGT) that our laboratory has engineered with twenty of the active site residues mutated in hAGT to those found in OGT, exhibited activity towards a broader range of substrates relative to native OGT. Among the substrates that the hOGT protein was found to act upon was interstrand cross-linked DNA connected by an alkylene linkage at the O6-position of dG to the complementary strand. In the present study the activity of hOGT towards DNA containing alkylene intrastrand cross-links (IaCL) at the O6- and O4-positions respectively of dG and dT, which lack a phosphodiester linkage between the connected residues, was evaluated. The hOGT protein exhibited proficiency at removal of an alkylene linkage at the O6-atom of dG but the O4-position of dT was refractory to protein activity. The activity of the chimeric hOGT protein towards these IaCLs to prepare well defined DNA–protein cross-linked conjugates will enable mechanistic and high resolution structural studies to address the differences observed in the repair adeptness of O4-alkylated dT by the OGT protein relative to other AGT variants.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...