Pd-Catalyzed tandem reaction of N-(2-cyanoaryl)benzamides with arylboronic acids: synthesis of quinazolines†
Abstract
The synthesis of 2,4-disubstituted quinazolines by a palladium-catalyzed reaction of arylboronic acids with N-(2-cyanoaryl)benzamides has been developed with moderate to excellent yields. The method shows good functional group tolerance. In particular, halogen and hydroxyl substituents, which are amenable for further synthetic elaborations, are well tolerated. Moreover, the present synthetic route could be readily scaled up to gram quantity without difficulty. The mechanism possibly involves nucleophilic addition to the nitrile function, forming an imine intermediate followed by an intramolecular addition to the amide and dehydration to the quinazoline ring.
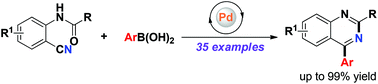
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...