Catalyst-free 1 : 2 annulation of quinolines with trifluoroacetylacetylenes: an access to functionalized oxazinoquinolines†
Abstract
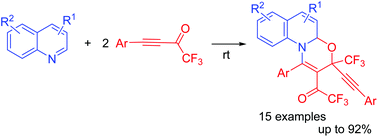
Metal- and solvent-free reaction of quinolines with two molecules of aryltrifluoroacetylacetylenes afforded 3-arylethynyl-3-trifluoromethyl-1,3-oxazinoquinolines in up to 92% yields. The formation of a zwitterionic intermediate in the first step triggered a multistep domino reaction. This one-pot synthesis opens an easy access to novel quinoline derivatives bearing trifluoromethyl, acetylene and ketone functions, thus providing a powerful tool for drug design.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...