Transition metal-free coupling of terminal alkynes and hypervalent iodine-based alkyne-transfer reagents to access unsymmetrical 1,3-diynes†
Abstract
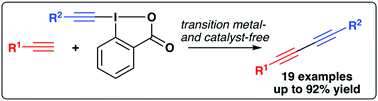
A variety of unsymmetrical 1,3-diynes can easily be accessed in good yields under catalyst- and transition metal-free conditions by reacting terminal alkynes with hypervalent iodine-based electrophilic alkyne-transfer reagents.


 Please wait while we load your content...
Please wait while we load your content...