Protic additives or impurities promote imine reduction with pinacolborane†
Abstract
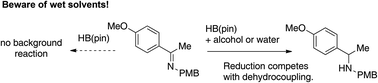
We report here that addition of stoichiometric amounts of alcohols or water to mixtures of imines and pinacolborane promote reduction reactions. The reactions of several imines were examined, revealing that alkyl imines were reduced, while aniline derived imines were not effectively reduced. The use of binol as an additive resulted in modest enantioinduction, however other chiral additives that were screened gave negligible enantioinduction. While the reactions described herein are not competitive in conversion with established imine reduction technologies, this work reveals that the presence of protic impurities must be considered as a promoter of side reactions in catalyzed imine hydroborations. Amines also promote imine reduction in certain cases, raising the possibility of a slow autocatalytic reaction. The ability of water or other protic impurities to promote the reduction of imines with pinacolborane represents an important identification of a potential source of background reaction in catalyzed reductions of imines.
- This article is part of the themed collection: New Talent


 Please wait while we load your content...
Please wait while we load your content...