Rearranged ergostane-type natural products: chemistry, biology, and medicinal aspects
Abstract
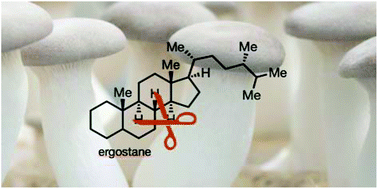
Classical steroids are long-known privileged leads in drug discovery. Their rearranged counterparts, though, have so far received less attention, although recent isolation and biological testing programmes have revealed a plethora of molecular entities that are both structurally intriguing, as well as biologically relevant. This review will highlight those natural products, and focus on ergostane-derived seco- and abeo-steroids. Their isolation, structure elucidation, and biological properties are reported. A special emphasis of this review lies in their respective (and typically proposed) biosyntheses, to help guide future bio-inspired synthetic attempts.
- This article is part of the themed collections: Chemical Biology in OBC and New Talent


 Please wait while we load your content...
Please wait while we load your content...