Direct defluorinative amidation–hydrolysis reaction of gem-difluoroalkenes with N,N-dimethylformamide, and primary and secondary amines†
Abstract
A novel and efficient method for the synthesis of arylacetamides by the reactions of gem-difluoroalkenes with N,N-dialkylformamides, and primary and secondary amines with the assistance of KOtBu and water was developed.
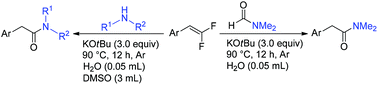
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...