A synthetic study toward the core structure of (−)-apicularen A†
Abstract
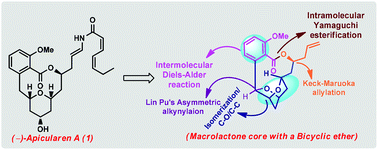
A concise synthetic strategy towards the core structure of (−)-apicularen A has been described in an 11-step synthetic sequence from a known intermediate. The key steps include tandem isomerization followed by C–O and C–C bond-forming reactions and iodocyclization strategies for the synthesis of a bicyclic ether embedded in the macrolactone ring. The applied reagent-controlled Keck–Maruoka allylation, Lin Pu alkynylation and Ricket–Diels–Alder reactions were used to simplify the synthetic sequence of related natural products. An intramolecular Yamaguchi lactonization constructed the macrolactone core, while the attempt to install the C11 hydroxyl chiral centre either under catalytic hydrogenation conditions or oxidative conditions was not successful.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...