A cascade synthesis of S-allyl benzoylcarbamothioates via Mumm-type rearrangement†
Abstract
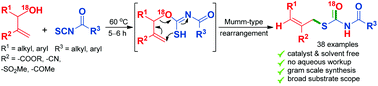
A catalyst and solvent free synthesis of S-allyl benzoylcarbamothioates has been achieved from the in situ generated benzoylcarbonimidothioates obtained by reacting MBH alcohols with aroyl isothiocyanates. An intramolecular thia-Michael addition of the in situ generated adduct triggers a Mumm-type rearrangement leading to a stereoselective synthesis of highly functionalised S-allyl benzoylcarbamothioates.
- This article is part of the themed collections: Synthetic methodology in OBC and Celebrating the RAOBC symposium


 Please wait while we load your content...
Please wait while we load your content...