Methyl 5-MeO-N-aminoanthranilate, a minimalist fluorogenic probe for sensing cellular aldehydic load†
Abstract
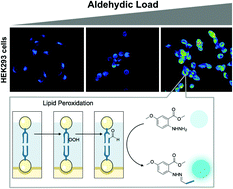
Methyl 5-MeO-N-aminoanthranilate, a fluorogenic probe comprising a single substituted benzene ring has been applied towards the fluorescence detection of endogenous carbonyls through rapid, catalyst-free complexation of these bio-derived markers of cell stress under physiological conditions. The products formed during the reaction between the probe and aldehydic products of lipid peroxidation, including malondialdehyde and long-chain aliphatic aldehydes relevant to the oxidative decomposition of cell membranes, have been evaluated. Live cell imaging of diethyl maleate-induced oxidative stress with or without pretreatment with α-tocopherol was carried out, with the result suggesting that the presented molecule might serve as a minimalist molecular probe capable of cellular “Aldehydic Load” detection by fluorescence microscopy. This work also outlines functional constraints of the fluorogenic probe (i.e. intramolecular cyclization), providing a realistic evaluation of methyl 5-MeO-N-aminoanthranilate for fluorescence-based aldehyde detection.
- This article is part of the themed collection: New Talent


 Please wait while we load your content...
Please wait while we load your content...