Synthesis of α-alkylated γ-butyrolactones with concomitant anhydride kinetic resolution using a sulfamide-based catalyst†
Abstract
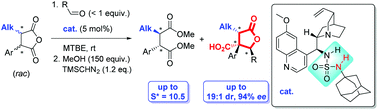
The Kinetic Resolution (KR) of α-alkylated enolisable disubstituted anhydrides has been shown to be possible for the first time. In the presence of an ad hoc designed novel class of bifunctional sulfamide organocatalyst, a regio-, diastereo- and enantioselective cycloaddition reaction between the enolisable anhydride and benzaldehydes provides densely functionalised γ-butyrolactones in one pot (up to 19 : 1 dr, 94% ee) with control over three contiguous stereocentres. The concomitant resolution of the starting material anhydride, provides access to a range of chiral succinate derivatives with selectivity factors up to S* = 10.5.


 Please wait while we load your content...
Please wait while we load your content...