Four-component acyloxy-trifluoromethylation of arylalkenes mediated by a photoredox catalyst†
Abstract
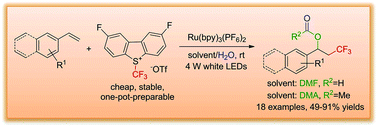
A four-component intermolecular trifluoromethylation–acyloxylation of arylalkenes induced by visible light has been developed in the presence of the photoredox catalyst Ru(bpy)3(PF6)2 under mild reaction conditions. A new Umemoto's reagent was used as a trifluoromethyl radical source, and this redox neutral reaction demonstrated good functional group tolerance for aryl alkenes with high yields up to 91%. The detailed reaction process was investigated based on control, deuterium and O18-labeling experiments to support that N,N-dimethylformamide (DMF)/H2O acted as an acyloxyl source.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...