Synthesis of chiral seven-membered β-substituted lactams via Rh-catalyzed asymmetric hydrogenation†
Abstract
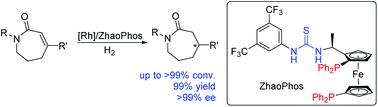
Rh/bisphosphine-thiourea ligand (ZhaoPhos)-catalyzed asymmetric hydrogenation of seven-membered β-substituted α,β-unsaturated lactams was successfully developed to prepare various chiral seven-membered β-substituted lactams with good to excellent results (up to >99% conversion, 99% yield, and >99% ee).
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...