One-step synthesis of N,N′-substituted 4-imidazolidinones by an isocyanide-based pseudo-five-multicomponent reaction†
Abstract
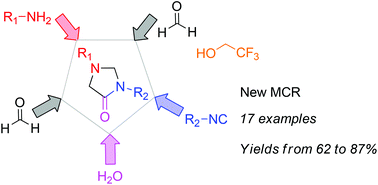
A pseudo-five-multicomponent reaction involving an isocyanide, a primary amine, two molecules of formaldehyde and water is reported, which gives N,N′-substituted 4-imidazolidinones when trifluoroethanol is used as the solvent. The reaction proceeds with good yields and with a wide variety of amines and isocyanides, providing an efficient new entry to these heterocycles. A preliminary study of the reaction mechanism suggests that trifluoroethanol, although acting as the solvent, is directly involved as a reagent in the reaction pathway.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...