Dimethylamine adducts of allylic triorganoboranes as effective reagents for Petasis-type homoallylation of primary amines with formaldehyde†
Abstract
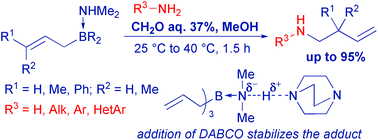
Dimethylamine adducts of triallyl-, triprenyl- and trans-cinnamyl(dipropyl)borane are effective reagents for mild homoallylation of primary amines with aqueous formaldehyde in MeOH without an inert atmosphere. A new concept is proposed for the explanation of the high stability of allylborane-amine adducts in aqueous MeOH.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...