Synthesis and Suzuki–Miyaura cross coupling reactions for post-synthetic modification of a tetrabromo-anthracenyl porphyrin†
Abstract
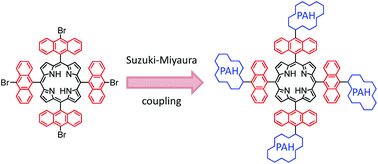
The outstanding properties of porphyrins and the extreme versatility of their synthesis and their functionalisation constitute real assets for the fabrication of opto- and electroactive materials or for biological applications. In the large collection of porphyrinic structures, meso-substituted anthracenylporphyrins are among the less studied. Here, we synthesised the 5,10,15,20-tetra-bromoanthracenylporphyrin (BrTAP) and we investigated its chemical reactivity by post-synthetic modification using Suzuki–Miyaura cross coupling reactions with a series of boronic acids to generate a collection of original tetra-anthracenyl porphyrin based molecules: tetraphenylanthracenylporphyrin (TPAP), tetratolylanthracenylporphyrin (TTAP), tetramethoxyphenylanthracenylporphyrin (TMPAP), tetranaphthylanthracenylporphyrin (TNAP) and tetrapyrenylanthracenylporphyrin (TPyAP). Optical characterisations of these modified porphyrins showed, in most cases, only emission of the porphyrin in the visible region with extinction of the fluorescence of PAHs in the UV or visible region.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...