Boronic acid-catalysed C-3 selective ring opening of 3,4-epoxy alcohols with thiophenols and thiols†
Abstract
In this protocol we described a boronic acid-catalysed C-3 selective ring opening of 3,4-epoxy alcohols with thiophenols and thiols as nucleophiles. This diastereo- and enantiospecific reaction provides an efficient entry to prepare a variety of hydroxyl sulfides. Through the directing effect of the hydroxyl group, nucleophilic attack on the C-3 position of the epoxide moiety is favoured. It can be rationalized in a proposed transition state, in which the boronic acid catalyst tethers both epoxides and S-nucleophiles.
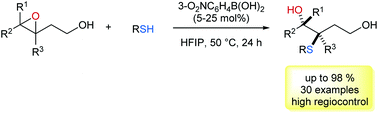
- This article is part of the themed collections: Catalysis & biocatalysis in OBC and New Talent


 Please wait while we load your content...
Please wait while we load your content...