Syntheses of the plant auxin conjugate 2-O-(indole-3-acetyl)-myo-inositol IAInos†
Abstract
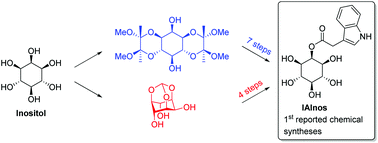
The plant hormone conjugate 2-O-(indole-3-acetyl)-myo-inositol (IAInos) has been selectively prepared for the first time by two routes from myo-inositol. One of the syntheses depended upon the construction of the 3-indoleacetyl group by a Fischer indole synthesis on an unreactive axial hydroxyl group, while the other via a direct acylation of the equatorially orientated hydroxy group created by conformational constraint of the cyclohexane ring. The latter synthesis produced IAInos in 5 steps and 29% overall yield.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...