Ruthenium(ii)-catalyzed synthesis of indazolone-fused cinnolines via C–H coupling with diazo compounds†
Abstract
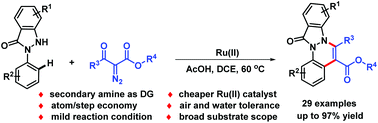
A robust, efficient and scalable method for the synthesis of 12H-indazolo[2,1-a]cinnolin-12-ones was developed. Significantly, a less developed cationic complex [Ru(p-cymene)(MeCN)3(SbF6)2] was found to be effective for this transformation. In this reaction, a tandem pathway of C–H ruthenation, Ru(II)–carbene formation, migratory insertion and condensation was involved. The results of a primary mechanistic study suggested that the C–H activation process might follow an electrophilic-type metalation/deprotonation mechanism.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...