Stereoselective synthesis of E-3-(arylmethylidene)-5-(alkyl/aryl)-2(3H)-furanones by sequential hydroacyloxylation-Mizoroki–Heck reactions of iodoalkynes†
Abstract
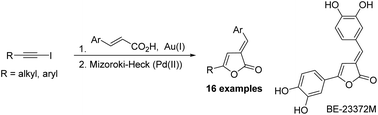
A modular, stereoselective synthesis of E-3-(arylidene)-5-(alkyl/aryl)-2(3H)-furanones was developed. The methodology features regioselective addition of β-aryl acrylic acids to iodoacetylenes to furnish the Z-acyloxy iodoalkenes. A stereoselective 5-exo-trig Mizoroki–Heck reaction of the acyloxy iodoalkenes generates the target E-2(3H)-furanones. The approach was applied in a formal synthesis of the naturally occurring kinase inhibitor BE-23372M.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...