Bioactive spiropyrrolizidine oxindole alkaloid enantiomers from Isatis indigotica Fortune†
Abstract
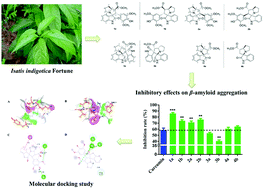
Four pairs of new spiropyrrolizidine oxindole enantiomers (1a/1b–4a/4b) were isolated from the leaves of Isatis indigotica Fortune. Their structures and absolute configurations were elucidated by a combination of NMR spectroscopic analyses, experimental and calculated electronic circular dichroism (ECD) and the assistance of quantum chemical predictions (QCP) of 13C NMR chemical shifts. Notably, all the isolated spiropyrrolizidine oxindoles are reported as natural products for the first time. The biosynthetic pathway of these unique structures was proposed to be formed by cycloaddition reaction. In addition, all the compounds were evaluated for their inhibitory effects on β-amyloid aggregation by ThT assay, and the optically pure compounds 1a/1b and 2a/2b exhibited better Aβ1–42 aggregation inhibition potency (85.8% and 73.6%, 71.5% and 75.8%, respectively) at a concentration of 20 μM, compared with the positive control curcumin (57.0%). The difference of the inhibitory pattern caused by chirality was also explained by molecular docking studies.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...