Synthesis of α-amino ketones through aminations of umpoled enolates†
Abstract
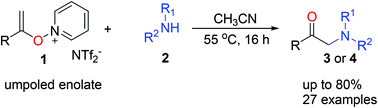
An efficient synthesis of α-amino ketones is developed using the umpolung strategy. Umpoled enolates such as N-alkenoxypyridinium salts react smoothly with diverse amines to give α-amino ketones via an SN2′ pathway. This umpolung strategy overcomes the drawbacks of traditional methods such as the need for prefunctionalized ketone derivatives. Our method also offers good chemical yields and high functional group tolerance.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...