Catalytic enantioselective one-pot approach to cis- and trans-2,3-diaryl substituted 1,5-benzothiazepines†
Abstract
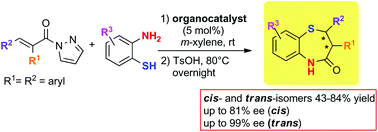
The first enantioselective catalytic approach to cis- and trans-2,3-diaryl substituted 1,5-benzothiazepines has been conveniently developed in a one-pot fashion, starting from α,β-unsaturated acyl pyrazoles and 2-aminothiophenol. The organocatalytic two-step sulfa-Michael/lactamization sequence is promoted by a readily available bifunctional thiourea and p-toluenesulfonic acid, respectively. The protocol enables access to both N-unprotected cis- and trans-diastereoisomers in moderate to satisfactory overall yields (up to 84%) and good to excellent ee values (up to 99%). Mechanistic investigations helped to shed light on the regio- and stereoselective outcome of the process.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...