Hydroarylation of unsaturated carbon–carbon bonds in cross-conjugated enynones under the action of superacid CF3SO3H or acidic zeolite HUSY. Reaction mechanism and DFT study on cationic intermediate species†
Abstract
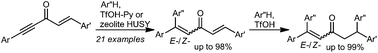
Reactions of cross-conjugated enynones,1,5-diarylpent-1-en-4-yn-3-ones, with arenes in the system TfOH-pyridine or under the action of acidic zeolite HUSY lead regioselectively to products of hydroarylation of the acetylene bond only,1,1,5-triarylpent-1,4-dien-3-ones, in yields up to 98%. These dienones add one more arene molecule to the double carbon–carbon bond in neat TfOH forming 1,1,5,5-tetraarylpent-1-en-3-ones in high yields. Cationic reaction intermediates have been studied by means of DFT calculations to elucidate plausible reaction mechanisms.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...