Stereo- and regioselective photocycloaddition of extended alkenes using γ-cyclodextrin†
Abstract
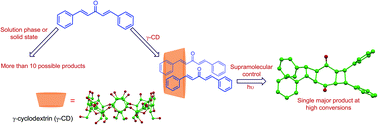
Photoexcitation of dibenzalacetones (1a–d) in homogeneous media and solid state yields a mixture of products with poor conversions. Irradiation of the reactants complexed to γ-cyclodextrin predominantly affords a single dimer (syn adduct 6) despite the possibility for several monomeric and dimeric products. High selectivity in the cavitand-mediated reaction along with the structural characterization of the inclusion complex provides insight into the supramolecular interactions that drive the self-assembly of the host–guest system.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...