tert-Butyl nitrite mediated nitrogen transfer reactions: synthesis of benzotriazoles and azides at room temperature†
Abstract
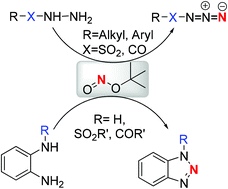
A conversion of o-phenylenediamines into benzotriazoles was achieved at room temperature using tert-butyl nitrite. The optimized conditions are also well suited for the transformation of sulfonyl and acyl hydrazines into corresponding azides. This protocol does not require any catalyst or acidic medium. The desired products were obtained in excellent yields in a short span of time.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...