FeCl3-promoted tandem 1,4-conjugate addition/6-endo-dig cyclization/oxidation of propargylamines and benzoylacetonitriles/malononitriles: direct access to functionalized 2-aryl-4H-chromenes†
Abstract
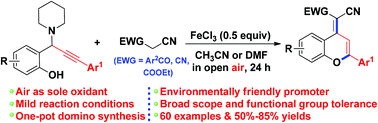
An efficient and concise procedure has been developed for the synthesis of functionalized 2-aryl-4H-chromenes based on a tandem reaction of propargylamines and benzoylacetonitriles/malononitriles in the presence of FeCl3 as an environmentally friendly promoter. This reaction involves a highly efficient tandem sequence consisting of 1,4-conjugate addition, 6-endo-dig cyclization, and oxidation. This protocol tolerates a variety of functional groups, thereby providing a practical and efficient method for the fabrication of 2-aryl-4H-chromene skeletons.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...