Synthesis of oxindoles through trifluoromethylation of N-aryl acrylamides by photoredox catalysis†
Abstract
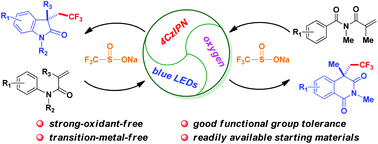
Mild and direct intramolecular oxidative aryltrifluoromethylations of activated alkenes have been established through visible light photocatalysis, affording a range of CF3-containing oxindoles or isoquinolinediones in the presence of an organic fluorophore-type photocatalyst 4CzIPN, oxygen and visible light irradiation under strong oxidant and transition metal free conditions. A variety of frequently encountered functional groups are well tolerated in this transformation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...