Purine-substituted imidazolium mesomeric betaines and their tautomeric N-heterocyclic carbenes. Formation of a cyclic borane adduct†
Abstract
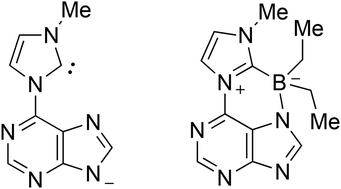
6-Chloropurine and 2,6-dichloropurine were reacted with N1-substituted imidazoles to give purin-6-yl substituted imidazolium salts, respectively. Deprotonation of the 1-methylimidazolium derivative resulted in the formation of the corresponding stable conjugated mesomeric betaine, whereas the 1-phenyl,- 1-vinyl- and 1-(2-hydroxyethyl) derivatives proved to be unstable. In situ generation of the mesomeric betaines by caesium carbonate in the presence of sulfur and selenium, however, gave the thiones and the selenones of the tautomeric purine-substituted imidazol-2-ylidene, respectively. Its anionic N-heterocyclic carbene was formally trapped by reaction with triethylborane at high temperatures as a cyclic boron adduct which is the first representative of a new heterocyclic ring system. DFT calculations gained insight into the electronic properties of the N-heterocyclic carbenes substituted by π-electron donators. Results of a single crystal X-ray analysis of the boron adduct are presented.
- This article is part of the themed collections: Synthetic methodology in OBC and Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...