Enantioselective syntheses and application of 4-epi-galiellalactone and the corresponding activity-based probe: from strained bicycles to strained tricycles†
Abstract
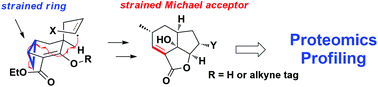
The [6,5,5] tricyclic fungal metabolite galiellalactone is a Michael acceptor that has been demonstrated to be a covalent inhibitor for Signal Transducer and Activator of Transcription 3 (STAT3). Recognizing the ring strain associated with the skeleton of this natural product, we utilized 1R-5S-bicyclo[3.1.0]hexan-2-one as the starting material and developed two novel approaches to accomplish the enantioselective total synthesis of the C4 epimer of galiellalactone in 5 and 7 steps, respectively, which capitalized on an efficient radical cyclization/fragmentation cascade reaction. Furthermore, an activity-based probe of 4-epi-galiellalactone with a terminal alkyne tag was successfully prepared to enable the experiments of activity-based protein profiling (ABPP). Through western blot and proteomic analysis, we not only confirmed the known target STAT3, but also identified a new target protein ataxin-7, which formed a covalent bond with the probe in intact cells via the Cys-129 residue.
- This article is part of the themed collections: Chemical Biology in OBC and New Talent


 Please wait while we load your content...
Please wait while we load your content...