First catalyst-free CO2 trapping of N-acyliminium ions under ambient conditions: sustainable multicomponent synthesis of thia- and oxazolidinyl carbamates†
Abstract
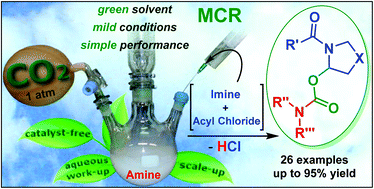
The first trapping of N-acyliminium ions by in situ generated carbaminic acid (product of carbon dioxide (CO2) and amine) is reported. This catalyst-free reaction provides a convenient and feasible approach to prepare N-acyl thia- and oxazolidinyl carbamates with good functional-group compatibility and high efficiency under green conditions. Furthermore, the multicomponent method features a broad substrate scope, facile product diversification, smooth scale-up and notable potential for polymer applications.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...