Phosphine-mediated partial reduction of alkynes to form both (E)- and (Z)-alkenes†
Abstract
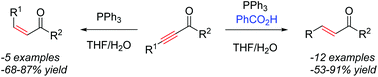
A mild, phosphine-mediated partial reduction of alkynyl carbonyls to the corresponding alkenes was developed. Tuning of the reaction conditions led to either the (E)- or (Z)-diastereomer with high selectivity. A range of alkynyl esters, amides, and ketones were reduced to form alkenes in good to high yields and with excellent functional group tolerance.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...