Solvent and catalyst free ring expansion of indoles: a simple synthesis of highly functionalized benzazepines†
Abstract
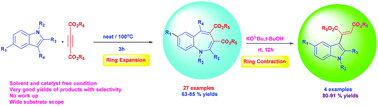
Highly functionalized benzazepines have been synthesized by using various substituted indoles and dialkylacetylene dicarboxylates under thermal and open air conditions. The reaction was carried out in a solvent and under catalyst free conditions and the products are formed in very good yields. The reaction proceeds in a concerted fashion thus providing potentially bioactive benzazepines by ring expansion of a five-membered indole ring. It is also described that the ring contraction of seven-membered benzazepines in the presence of KOtBu base at room temperature smoothly afforded the corresponding 3-alkenylated indole derivatives in very good yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...