Synthesis and properties of dithienylethene-functionalized switchable antibacterial agents†
Abstract
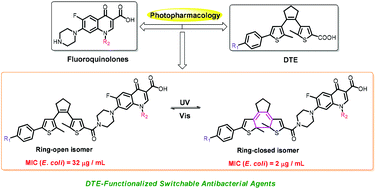
Photopharmacology involving azobenzene has offered a viable alternative for combating bacterial resistance. However, the degradation and potential toxicity of azobenzene limit its further study in vivo. Therefore, searching for an appropriate photoswitch for further clinical application is highly desirable. Herein a series of dithienylethene-functionalized switchable antibacterial agents have been designed and prepared by the introduction of the dithienylethene scaffold into fluoroquinolones. And it was found that these switchable antibacterial agents displayed good photochromism and fluorescence switching behaviors upon irradiation with UV/Vis light in DMSO. Surprisingly, methoxy-substituted dithienylethenes 3a and 3b exhibited fluorescence turn-on behavior. Furthermore, it was found that all of the open-isomers showed partial antibacterial activity on E. coli and S. aureus compared with the native drugs. Apart from 2a and 2b, the other switchable antibacterial agents showed a large difference in antibacterial activity on Gram-negative E. coli between the open and closed forms, in which the antimicrobial activity of the ring-closed isomers for 1b and 3b was 16 times that of the corresponding ring-open isomers. DFT calculations showed that the ring-closed isomers of 1b and 3b presented a rigid “S-type” conformation, which may be conducive to forming more stable complexes with the DNA gyrase of E. coli.
- This article is part of the themed collections: Chemical Biology in OBC and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...