Regioselective N-1 and C-2 diacylation of 3-substituted indoles with arylglyoxal hydrates for the synthesis of indolyl diketones†
Abstract
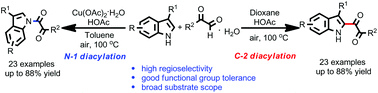
A highly regioselective N-1 and C-2 diacylation of 3-substituted indoles with arylglyoxal hydrates to afford N-1 and C-2 indolyl diketones in moderate to good yields is described. Notably, the control of regioselectivity is achieved by small changes in the Cu catalyst, additive and solvent. Importantly, the intermediates for N-1 and C-2 diacylation were detected and two plausible pathways were also proposed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...