Organocatalysis and catalyst aggregation: a study using the asymmetric synthesis of benzofuranones as a test reaction†
Abstract
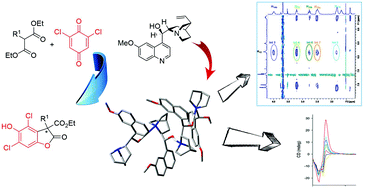
A common problem encountered in enantioselective organocatalysis is the aggregation of the catalyst, which can result in a relevant decrease of the efficiency and selectivity of the process. In the asymmetric synthesis of chiral benzofuranones, recently reported by us, we noted a remarkable increase of the reaction yield upon the addition of one of the reagents in a portionwise manner rather than in a single addition. We investigated this phenomenon by several experimental techniques such as 1D and 2D NMR experiments, UV-Vis spectroscopy, circular dichroism and dynamic light scattering. In addition, we studied the kinetic profile of this reaction using a simple numerical model and carried out in silico investigations. All these different approaches point to the conclusion that in the reaction medium a supramolecular polymerization/aggregation phenomenon, based on weak interactions, occurs and such a process is promoted by a quinone, which is one of the reagents of the benzofuranone synthesis. The portionwise mode of addition is a known strategy which can improve the performance of many synthetic procedures and this strategy is commonly adopted on account of empirical experience. However, our results provide an explanation, based on a chemical kinetic model, of the reason why the portionwise addition affects in such a dramatic way the yield of the benzofuranone synthesis catalyzed by Cinchona alkaloids.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...