Switching a regular tryptophan C4-prenyltransferase to a reverse tryptophan-containing cyclic dipeptide C3-prenyltransferase by sequential site-directed mutagenesis†
Abstract
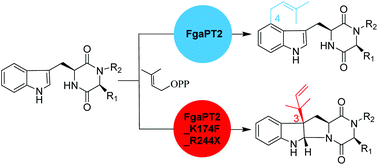
FgaPT2 from Aspergillus fumigatus catalyzes a regular C4- and its mutant K174A a reverse C3-prenylation of L-tryptophan in the presence of dimethylallyl diphosphate. FgaPT2 also uses tryptophan-containing cyclic dipeptides for C4-prenylation, while FgaPT2_K174A showed almost no activity toward these substrates. In contrast, Arg244 mutants of FgaPT2 accept very well cyclic dipeptides for regular C4-prenylation. In this study, we demonstrate that FgaPT2_K174F, which catalyzes a regular C3-prenylation on tyrosine, can also use cyclo-L-Trp-L-Ala, cyclo-L-Trp-L-Trp, cyclo-L-Trp-Gly, cyclo-L-Trp-L-Phe, cyclo-L-Trp-L-Pro, and cyclo-L-Trp-L-Tyr as substrates, but only with low activity. Combinational mutation on Lys174 and Arg244 increases significantly the acceptance of these cyclic dipeptides. With the exception of cyclo-L-Trp-L-Trp, the tested dipeptides were much better accepted by FgaPT2_K174F_R244X (X = L, N, Q, Y) than FgaPT2, with an increase of two- to six-fold activity. In comparison to FgaPT2_K174F, even two- to ten-fold conversion yields were calculated for the double mutants. Isolation and structural elucidation of the enzyme products revealed stereospecific reverse C3-prenylation on the indole ring, resulting in the formation of syn–cis configured hexahydropyrroloindole derivatives. The results presented in this study highlight the convenience of site-directed mutagenesis for creating new biocatalysts.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...