Development of triazine-based esterifying reagents containing pyridines as a nucleophilic catalyst†
Abstract
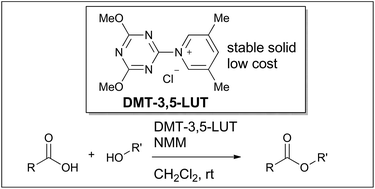
We have developed new triazine-based esterifying reagents comprising pyridines that can act as a nucleophilic catalyst. 1-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-3,5-lutidinium chloride (DMT-3,5-LUT) was found to exhibit a superior reactivity for the dehydrating condensation reaction between carboxylic acids and alcohols. The reaction of DMT-3,5-LUT with carboxylic acids produces intermediacy of acyloxytriazines, which is known to exhibit moderate reactivity toward alcohols, with concomitant liberation of 3,5-lutidine. The subsequent chemical transformation of the acyloxytriazines and alcohols into esters can be accelerated by the action of 3,5-lutidine as a nucleophilic catalyst. The detailed reaction mechanism revealed by a time-course analysis of the reactions is also discussed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...