Biosurfactant-functionalized porphyrin chromophore that forms J-aggregates†
Abstract
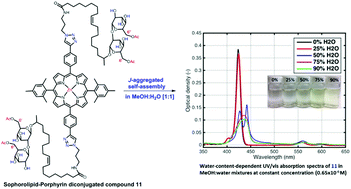
Structurally complex biosynthesized building blocks whose structures can be systematically varied are of great interest for the synthesis of manipulable self-organizing supramolecular systems. Sophorolipids (SLs) are an important class of glycolipid biosurfactants that consists of a sophorose (glucose disaccharide) polar head group that allows structural diversification by full or selective acetylation at the 6′- and 6′′-positions. Porphyrins are a group of naturally-occurring heterocyclic macromolecular organic compounds that have efficient charge transfer properties. Herein we describe the synthesis of SL–porphyrin conjugates where the number of sophorolipid arms, availability of hydrogen bonding sophorose hydroxyl groups and rigidity of the lipid chain were systematically varied. SLs differing in ‘sophorose acetylation’ and ‘lipid unsaturation’ were conjugated to zinc-porphyrin dyes by copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) ‘click’ chemistry. Mono-, di-, and tetra-conjugation of SL-arms to the zinc-porphyrin core provided variation in SL-arm steric effects. UV-vis spectra in methanol/water reveal features indicative of supramolecular J-type aggregates. The synthesized compounds were designed to provide a library of unique bio-based molecules with built-in variation in non-covalent interactions, hydrogen-bonding, π–π stacking, metal–ligand coordination, dipole–dipole, van der Waals, and hydrophobic interactions for future interrogation of supramolecular self-assembly into functional materials for electro-optical applications.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...