Experimental evidence for the formation of cationic intermediates during iodine(iii)-mediated oxidative dearomatization of phenols†
Abstract
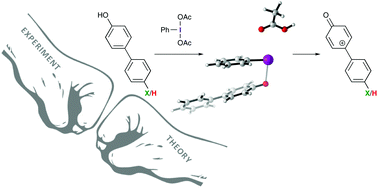
Iodine(III)-based oxidants are commonly used reagents for the oxidative dearomatization of phenols. Having a better understanding of the mechanism through which these reactions proceed is important for designing new iodine(III)-based reagents, catalysts, and reactions. We have performed a Hammett analysis of the oxidative dearomatization of substituted 4-phenylphenols. This study confirms that iodine(III)-mediated oxidative dearomatizations likely proceed through cationic phenoxenium ions and not the direct addition of a nucleophile to an iodine-bound phenol intermediate.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...