Iodine-mediated cross-dehydrogenative coupling of pyrazolones and alkenes†
Abstract
An iodine-mediated alkenylation of pyrazolones with simple alkenes under an air atmosphere has been developed. By this protocol, the pharmaceutically relevant pyrazolone scaffold was directly adorned with a readily transformable olefinic functional group through a cross-dehydrogenative coupling process in moderate to excellent yields. The coupling products could be facilely manipulated by, for example, the Heck reaction and hydrogenation treatment, thus enriching the pyrazolone derivatives. Based on the observation of the reaction intermediate and mechanistical experiments, two possible reaction pathways were proposed.
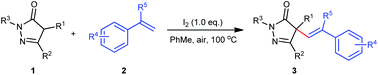
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...