Native chemical ligation at methionine bioisostere norleucine allows for N-terminal chemical protein ligation†
Abstract
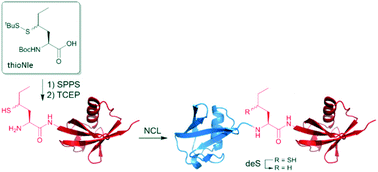
The development of γ-thionorleucine (ThioNle) as a handle for native chemical ligation–desulfurization is reported here. ThioNle is a new addition to the expanding thiolated amino acid toolbox and serves as a methionine substitute in NCL with the advantage that it lacks the undesirable oxidation-prone thioether moiety. Its usefulness for N-terminal ubiquitination is demonstrated by efficient preparation of fully synthetic linear diubiquitin with preserved protein folding compared to the expressed material. Interestingly, gel-based deubiquitinating assays revealed that the methionine to norleucine substitution did affect diubiquitin cleavage, which may indicate a more profound role for methionine in the interaction between ubiquitin and the deubiquitinating enzymes than has been known so far.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...